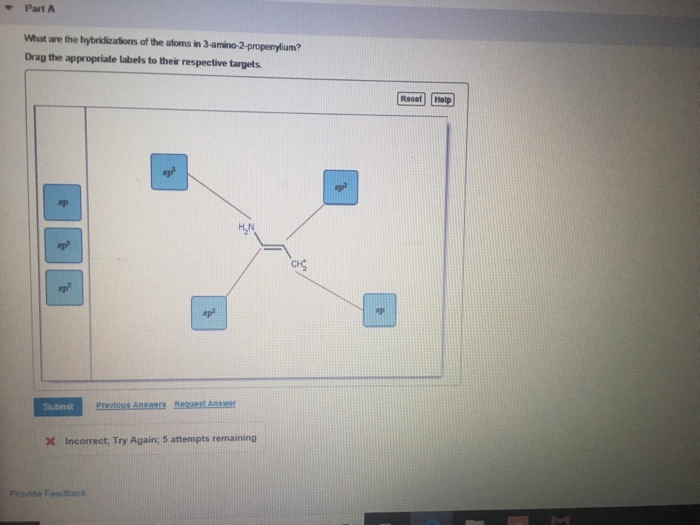
What Are The Hybridizations Of The Atoms In 3 Amino 2 Propenylium
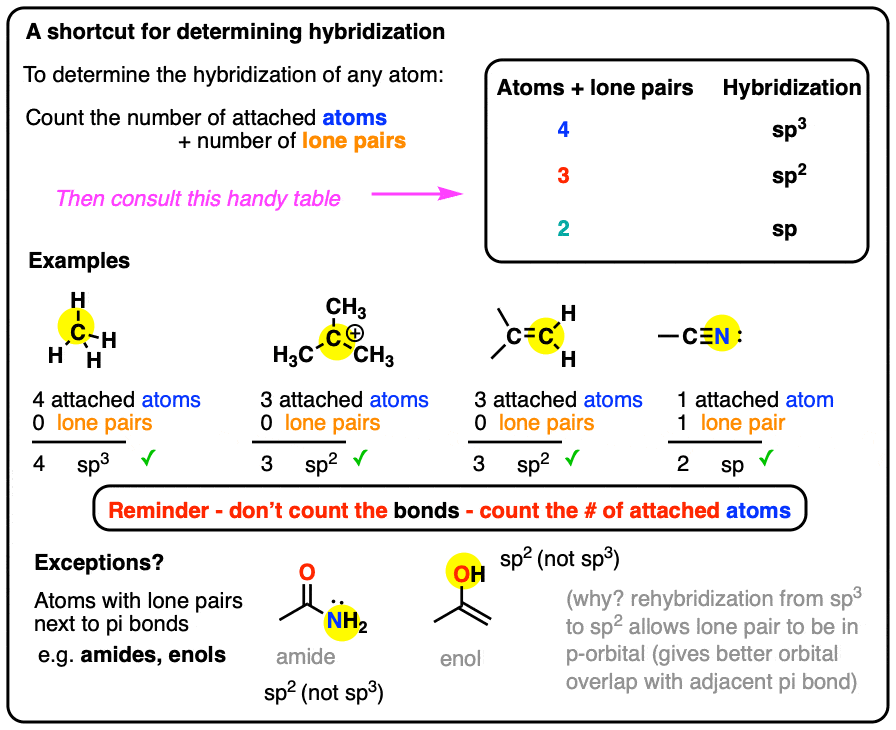
What Are The Hybridizations Of The Atoms In 3 Amino 2 Propenylium - If there are 3 regions of electron density, the atom is sp2 hybridized. If there are 2 regions of electron density, the atom is sp hybridized. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Drag the appropriate labels to. To put it plain, i can summarize the hybridizations in the following picture: There are 2 steps to solve this one. So, the 3 groups around the central atom gives you the sp 3. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule.
8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. There are 2 steps to solve this one. To put it plain, i can summarize the hybridizations in the following picture: So, the 3 groups around the central atom gives you the sp 3. If there are 3 regions of electron density, the atom is sp2 hybridized. If there are 2 regions of electron density, the atom is sp hybridized. Drag the appropriate labels to. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If there are 3 regions of electron density, the atom is sp2 hybridized. There are 2 steps to solve this one. If there are 2 regions of electron density, the atom is sp hybridized. Drag the appropriate labels to. To put it plain, i can summarize the hybridizations in the following picture: 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. So, the 3 groups around the central atom gives you the sp 3.
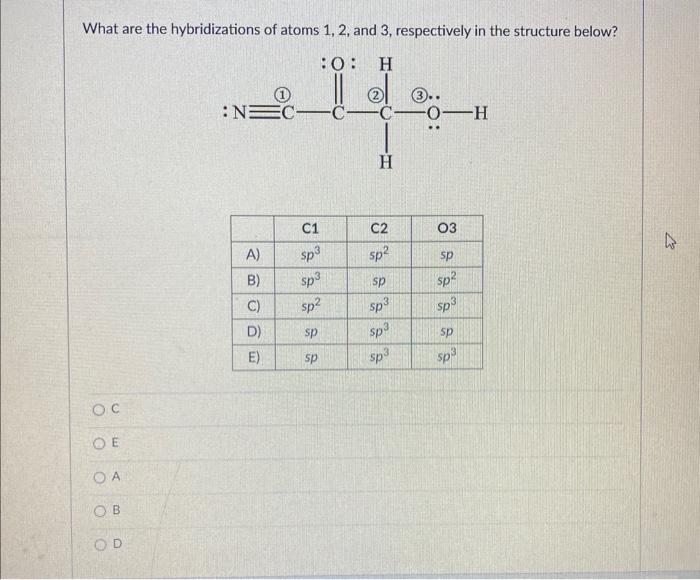
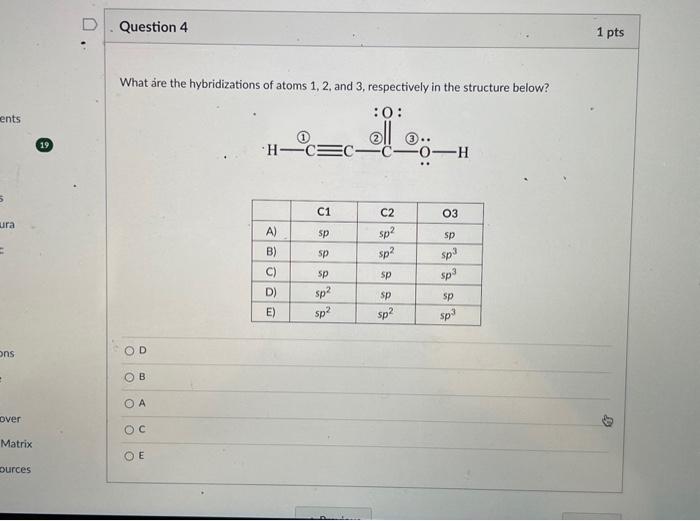
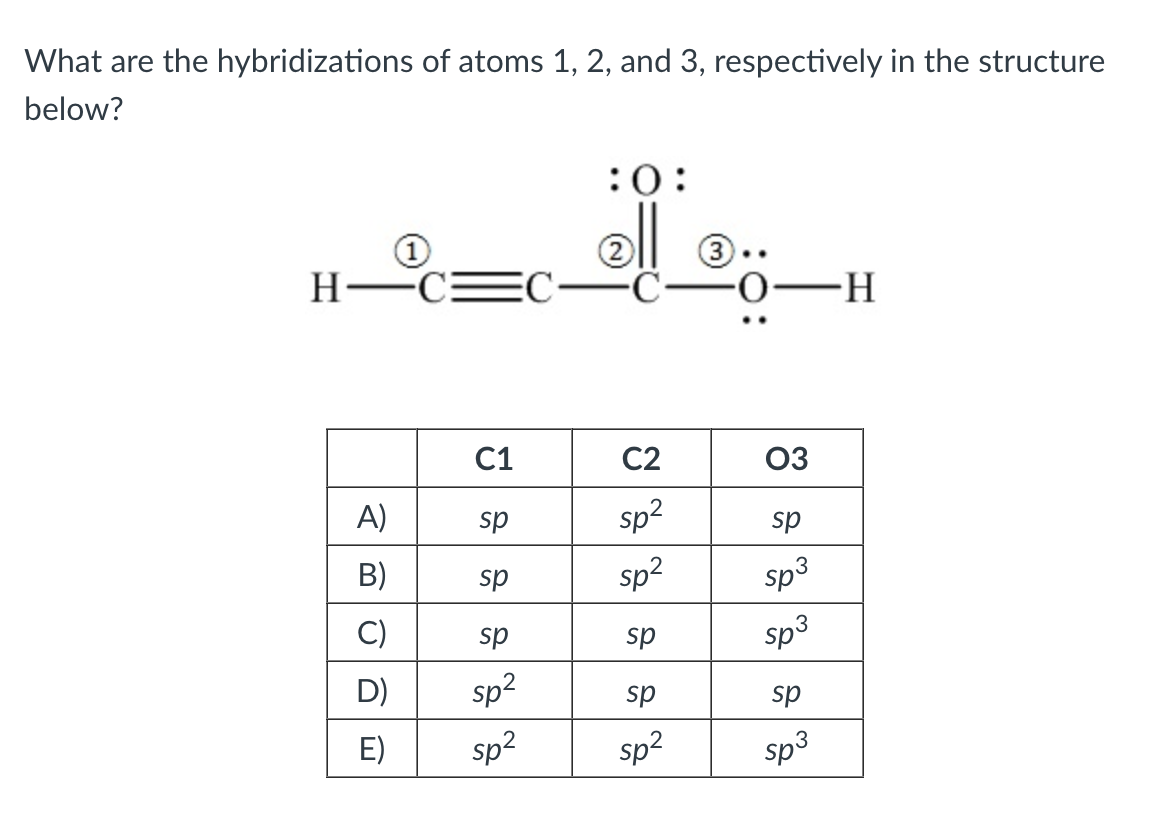
Solved What are the hybridizations of atoms 1,2 , and 3 ,
To put it plain, i can summarize the hybridizations in the following picture: If there are 2 regions of electron density, the atom is sp hybridized. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Drag the appropriate labels to. 8 rows the shapes of organic molecules may be understood by looking at.
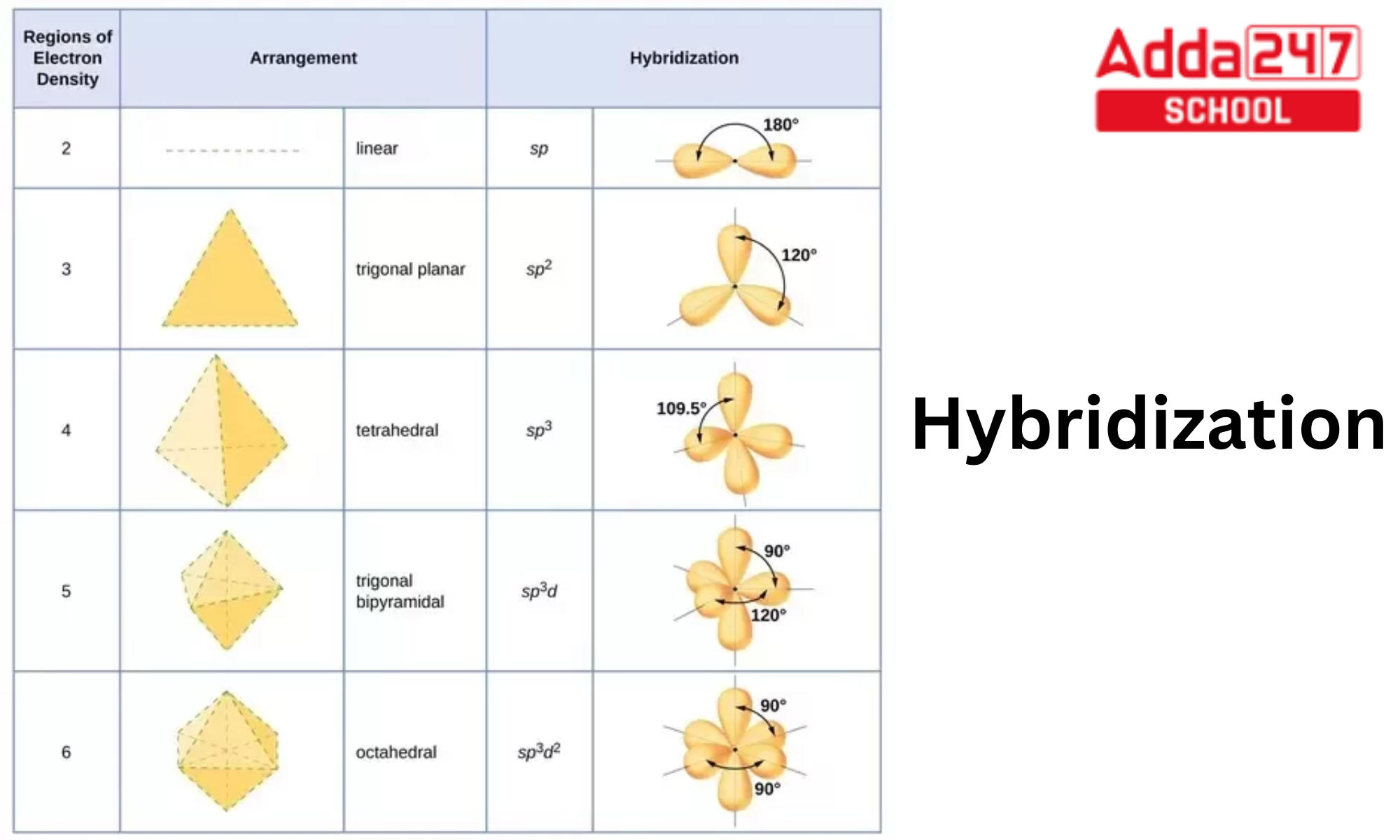
What is Hybridization? sp3, sp2, Examples and Formula
So, the 3 groups around the central atom gives you the sp 3. Drag the appropriate labels to. If there are 3 regions of electron density, the atom is sp2 hybridized. To put it plain, i can summarize the hybridizations in the following picture: If there are 2 regions of electron density, the atom is sp hybridized.
what are the hybridizations of atoms 1 2and 3 respectively in the
If there are 3 regions of electron density, the atom is sp2 hybridized. To put it plain, i can summarize the hybridizations in the following picture: 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. There are 2 steps to solve this one. Drag the.
Types of Hybridization Definitions, Examples, Key Features, Steps to
So, the 3 groups around the central atom gives you the sp 3. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. There are 2 steps to solve this one. If there are 3 regions of electron density, the atom is sp2 hybridized. If there.
Solved I posted this question once before and they got it
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If there are 3 regions of electron density, the atom is sp2 hybridized. Drag the appropriate labels to. To put it plain, i can summarize the hybridizations in the following picture: There are 2 steps to solve this one.
Solved What are the hybridizations of atoms 1,2 , and 3 ,
If there are 3 regions of electron density, the atom is sp2 hybridized. Drag the appropriate labels to. There are 2 steps to solve this one. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. If there are 2 regions of electron density, the atom.
Solved What are the hybridizations of atoms 1,2 , and 3 ,
If there are 3 regions of electron density, the atom is sp2 hybridized. There are 2 steps to solve this one. Drag the appropriate labels to. If there are 2 regions of electron density, the atom is sp hybridized. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Hybridization Orbitals Chart
To put it plain, i can summarize the hybridizations in the following picture: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. So, the 3 groups around the central atom gives you the sp 3. If there are 2 regions of electron density, the atom is sp hybridized. 8 rows the shapes of.
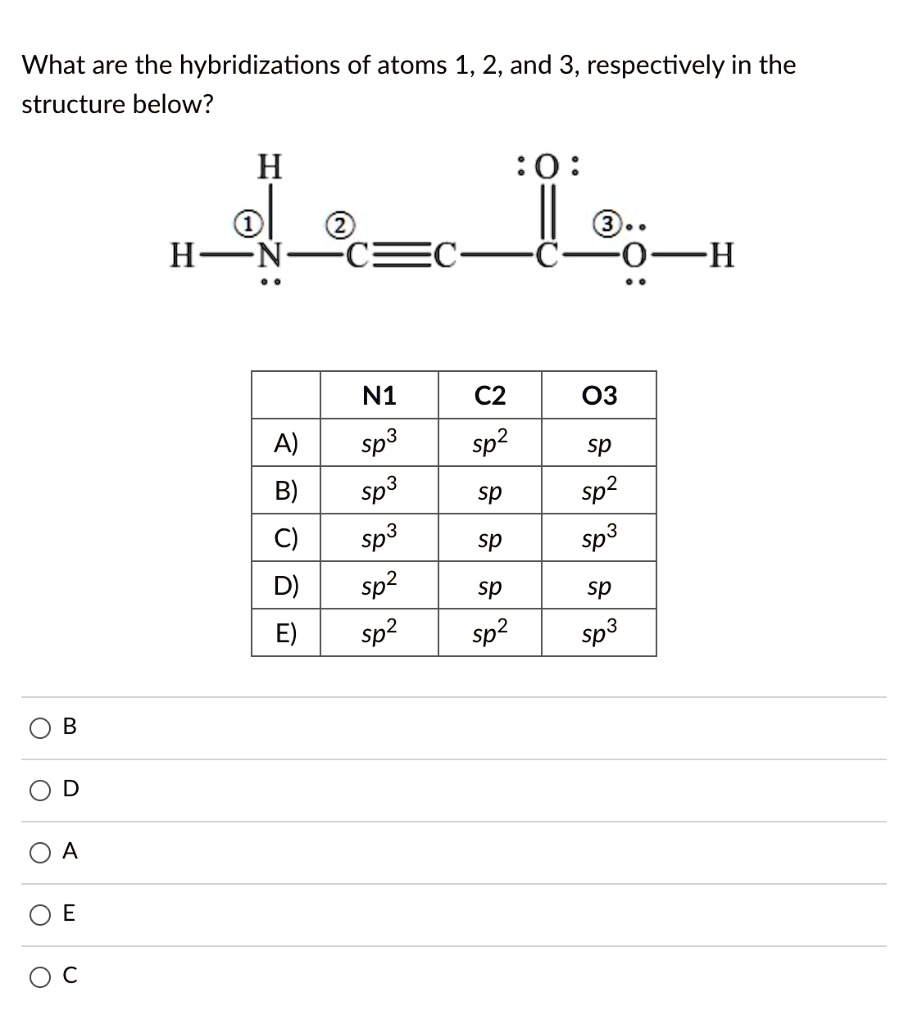
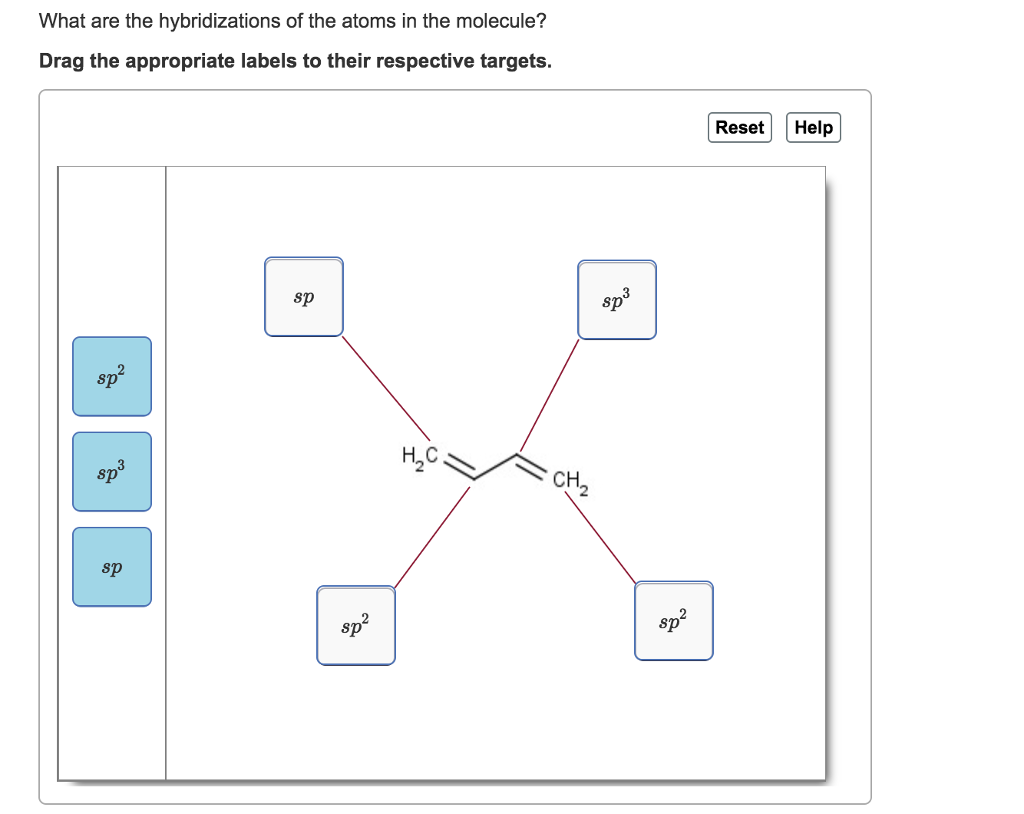
Solved What are the hybridizations of the atoms in the
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. To put it plain, i can summarize the hybridizations in the following picture: 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. If there are 2 regions of electron.
میانبر استاد شیمی آلی تولیدی فرمیک
To put it plain, i can summarize the hybridizations in the following picture: Drag the appropriate labels to. There are 2 steps to solve this one. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. You'll get a detailed solution from a subject matter expert.
There Are 2 Steps To Solve This One.
Drag the appropriate labels to. So, the 3 groups around the central atom gives you the sp 3. If there are 3 regions of electron density, the atom is sp2 hybridized. To put it plain, i can summarize the hybridizations in the following picture:
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
If there are 2 regions of electron density, the atom is sp hybridized. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule.